

Since molarity tells yo uthe number of moles of solute you get per liter of solution, you can say that the molarity of the chloride anions will be #0.01996 color(red)(cancel(color(black)("moles MgCl"_2))) * (color(red)(2)color(white)(a)"moles Cl"^(-))/(1color(red)(cancel(color(black)("mole MgCl"_2)))) = "0.03392 moles Cl"^(-)#Īt this point, all you have to do is figure out how many moles of chloride anions you get in #"1 L"# of this solutions by suing the fact that #"250 mL"#, the equivalent of #1/4"th"# of a liter, contains #0.03992# moles
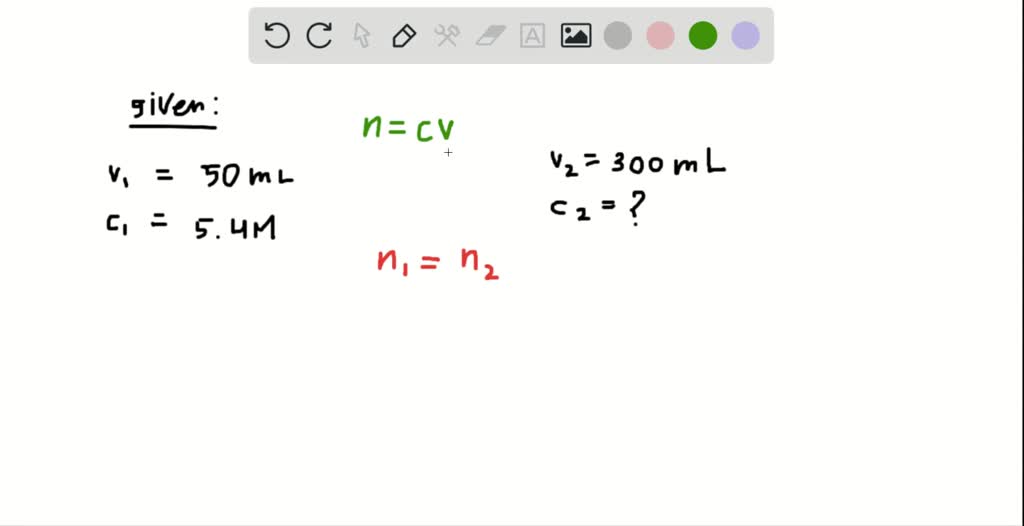

What is the uncertainty in the volume delivered, A solution containing NaCl was made by dissolving 7.5 ± 0.1 g of NaCl in a 250.00 ± 0. Now, each mole of magnesium chloride will produce #color(red)(2)# moles of chloride anions in solution Answer: 2 on a question Which of the following measurements is expressed in three significant figures a. Write each answer with the correct number of significant figures and find the absolute uncertainty. To do that, use the compound's molar mass, which essentially tells you the mass of one mole of magnesium chloride How do you draw two five carbon rings that share an atom 5. write a equivalency statement relating candelas and teracondelas. The first thing to do here is figure out how many moles of magnesium chloride were needed to make this solution. To how many decimal places should you report the volume (in mL) contained in a 250 mL volumetric fla 2. Since magnesium chloride is a soluble ionic compound, this implies that every mole of the salt that dissolves in water will produce #1# mole of magnesium cations and #color(red)(2)# moles of chloride anions. The trick here is to realize that one formula unit of magnesium chloride, #"MgCl"_2#, contains one magnesium cation, #"Mg"^(2+)#, and two chloride anions, #"Cl"^(-)#.


 0 kommentar(er)
0 kommentar(er)
